Ordered, immediately called back and the same day delivered the order.Very pleased with the work. Thank you for prompt and accurate work https://africarx.co.za/buy-levitra-south-africa.html great prices, delivered on the day of the order. Pleasant managers consult by phone.
Pii: s0753-3322(02)00291-3
Dossier: Free amino acids in human health and pathologies
Arginine and immunity: a unique perspective
Carmelo Nieves Jr, Bobbi Langkamp-Henken *
Food Science and Human Nutrition Department, University of Florida, PO Box 110370, Gainesville, FL 32611-0370, USA
Abstract
Arginine functions in the body as a free amino acid, a component of most proteins, and the substrate for several non-protein,
nitrogen-containing compounds, many of which function in immunity. Although arginine is synthesized in the body, it is not made insufficient quantities to support growth or meet metabolic requirements during periods of stress. Based on the biochemical and physiologicalrole of arginine in maintaining health and immunity, arginine is being added at pharmacologic concentrations to enteral formulas to boostimmune function. Unfortunately, animal and human studies that investigate enteral arginine supplementation as the single variable do notshow clear immunologic benefit. The inconsistent effects of arginine supplementation on immune function are due to numerous factors, suchas the amount and timing of arginine supplementation, the animal species or strain of species, and the experimental model. Systematic studyis required to determine whether a basal dietary intake of arginine is required to maintain immune function during health and how mucharginine is required to meet metabolic requirements during periods of growth or stress. 2002 Éditions scientifiques et médicales ElsevierSAS. All rights reserved.
Keywords: Arginine; Immune function; Arginase; Nitric oxide
1. Introduction
2. Arginine metabolism
Arginine functions in the body as a free amino acid, a
Classification of arginine as an essential or non-essential
component of most proteins, and as the substrate for several
amino acid has been difficult. By the classical definition,
non-protein, nitrogen-containing compounds. As a free
essential amino acids cannot be synthesized in the body to
amino acid, arginine functions as an intermediate in the urea
meet the needs for optimal growth. In 1930, Scull and Rose
cycle. As one of the 20 common α-amino acids, arginine is
observed that arginine was synthesized in vivo, and classi-
an integral component of mammalian proteins. As a sub-
fied arginine as a non-essential amino acid . Nitrogen
strate for several non-protein, nitrogen-containing com-
balance studies, which are currently used to classify an
pounds, arginine indirectly participates in the rapid regen-
amino acid as essential or non-essential for humans, also
classified arginine as non-essential . Some con-
vasodilatation, neurotransmission, calcium release, and ulti-
sider this to be an inaccurate evaluation and argue that
mately immunity. This review will briefly address the
arginine may need to be reclassified as a semi-essential or
biochemical basis for arginine in health and disease and
conditionally essential amino acid (reviewed in ).
discuss animal and human studies available in the literature
Support for this argument comes from animal studies (rats
that investigate the role of arginine supplementation on
and dogs) in which sub-optimal weight gain is observed
when arginine is excluded from the diet during growthUnder these conditions (growth) in vivo synthe-sis of arginine is not sufficient, hence the term conditionallyessential. During times of physiologic stress, arginine syn-
thesis may not be able to keep up with metabolic demands.
E-mail address: rjhenken@mail.ifas.ufl.edu (B. Langkamp-Henken).
2002 Éditions scientifiques et médicales Elsevier SAS. All rights reserved.
PII: S 0 7 5 3 - 3 3 2 2 ( 0 2 ) 0 0 2 9 1 - 3
C. Nieves Jr, B. Langkamp-Henken / Biomed Pharmacother 56 (2002) 471–482
This is another ‘condition’ where arginine may become
virtually all tissues including the kidney, brain, liver, skel-
etal muscle and lungs However, the purpose of this
One of the major functions of arginine within the body is
as an intermediate in the urea cycle. The free amino acid
Another function of arginine is protein synthesis. Argin-
arginine contains a guanidino group, which is essential for
ine can be converted into proline, glutamate and glutamine
the synthesis of urea in most mammals. Urea is primarily
(see all of which are common amino acids found
synthesized in the liver and excreted by the kidneys. In the
within most proteins. Synthesis of these three amino acids
cytosol of hepatocytes, arginase-I removes the guanidino
begins with arginine being converted into ornithine. Orni-
group from arginine to produce urea and ornithine (see
thine is then converted into pyrroline-5-carboxylate. The
Urea is then transported from the hepatocyte into the
enzyme for this reaction is ornithine aminotransferase.
bloodstream and ornithine is used to regenerate arginine
Pyrroline-5-carboxylate can be converted into either proline
within the hepatocyte. It is important to note that a second
using pyrroline-5-carboxylate reductase or in two-steps
form of arginase (arginase-II) exists and is expressed in
converted into glutamyl-γ-semialdehyde, then glutamate.
Fig. 1. Diagram of arginine metabolites. Metabolites are bolded and enzymes/proteins are italicized.
Abbreviations: ADC, arginine decarboxylase; A:GAT, arginine:glycine amidinotransferase; DAO, diamine oxidase; Glu synthase, glutamine synthase; GMT,guanidinoacetate-
N -methyltransferase; NOS-1, nitric oxide synthase-1; NOS-2, nitric oxide synthase-2; NOS-3, nitric oxide synthase-3; OAT, ornithineaminotransferase; ODC, ornithine decarboxylase; P-5-C dehydrogenase, pyrroline-5-carboxylate dehydrogenase; P-5-C reductase, pyrroline-5-carboxylatereductase; and PT, polyamine transporter.
C. Nieves Jr, B. Langkamp-Henken / Biomed Pharmacother 56 (2002) 471–482
The conversion of pyrroline-5-carboxylate into glutamyl-γ-
arginine indirectly provide the substrate for polyamine
semialdehyde is spontaneous and requires no enzyme;
synthesis, it also indirectly stimulates polyamine synthesis
however, conversion of glutamyl-γ-semialdehyde into
by stimulating the release of growth hormone . In
glutamate requires the enzyme pyrroline-5-carboxylate de-
turn, growth hormone stimulates the release of insulin-like
hydrogenase. Synthesis of glutamine from glutamate is
growth factor-1, which stimulates polyamine synthesis by
catalyzed by glutamine synthase and requires ammonia. In
increasing ornithine decarboxylase activity .
addition to protein formation, glutamine prevents the accu-
While ornithine decarboxylase is the rate-limiting en-
mulation of ammonia in extrahepatic tissue by transporting
zyme in polyamine production a second enzyme, arginine
ammonia through the bloodstream to the hepatocytes for
decarboxylase, also synthesizes polyamines In a study
conducted in rats, arginine decarboxylase was shown to
Arginine metabolism also generates several essential
decarboxylate ornithine (
K = 0.25 mM) to produce pu-
non-protein, nitrogen-containing compounds. Some ex-
trescine or to decarboxylate arginine (
K = 0.75 mM) to
amples of these compounds are creatine, polyamines, agma-
produce agmatine . Moreover, both human and animal
tine and nitric oxide. Creatine is primarily synthesized in the
studies have shown that the enzyme agmatinase can use
liver and transported through the blood stream to muscle
agmatine as a substrate to produce putrescine
tissue. Creatine functions as a carrier for phosphate and is
In addition to being a substrate for polyamine synthesis,
needed for the rapid regeneration of adenosine triphosphate
agmatine also regulates intracellular concentrations of
in the muscles. Synthesis of creatine is dependent on the
polyamines. Regulation of intracellular polyamine produc-
guanidino group of arginine and is a two-step reaction (see
tion is important because high levels of polyamines are
). In the first reaction arginine:glycine amidinotrans-
toxic to cells (reviewed in Agmatine regulates
ferase, transfers the guanidino group from arginine onto
polyamine production by decreasing ornithine decarboxy-
glycine, this produces guanidinoacetate and ornithine. Next,
lase activity and enhancing the transcription of antizyme
the enzyme guanidinoacetate
N -methyltransferase transfers
. Antizyme is an endogenous protein that decreases
a methyl group from
S -adenosylmethionine to guanidinoac-
intracellular polyamine synthesis through two different
etate. Products from this reaction are creatine and
mechanisms. First, antizyme decreases ornithine decarboxy-
lase activity and second, antizyme accelerates the degrada-
(phosphocreatine) occurs at the guanidino group in the
tion of ornithine decarboxylase . Although,
regulating the production polyamines decreases intracellular
Approximately 10% of arginine in the plasma is used to
polyamines levels, it does not prevent the uptake of
synthesize creatine, even though creatine is regenerated
polyamines from extracellular sources via the polyamine
when phosphocreatine phophorylates adenosine diphos-
transporter. Another function of agmatine and antizyme is to
phate . This is due to the fact that phosphocreatine
decrease the activity of the polyamine transporter, thus
undergoes spontaneous degradation to creatinine. Creatinine
limiting the uptake of extracellular polyamines
cannot be degraded by mammals and is excreted by the
In addition to regulating polyamine levels, agmatine and
kidneys. A study conducted by Cockroft and Gault esti-
arginine also regulate production of nitric oxide. Nitric
mated creatinine excretion to be 23.6 ± 5 mg/kg over 24 h in
oxide is an antimicrobial agent that is effective against
males 18–29 years old . Using a 70 kg male as a
intracellular pathogens, extracellular parasites and bacteria
reference, 2.0–3.1 g of arginine is needed daily to replace
Nitric oxide is also a neurotransmitter (reviewed
creatine loss. A typical gram of dietary protein provides
in and vasodilator . The enzyme that produces
54 mg of readily available arginine If our reference
nitric oxide is nitric oxide synthase. The substrate for this
male consumes the recommended daily allowance of 0.8 g
reaction is arginine and the products are nitric oxide and
of protein per kg of body weight, this supplies 3.0 g of
citrulline (see There are three isoforms of nitric
arginine. This means that the dietary intake of arginine may
oxide synthase; these are NOS-1, NOS-2 and NOS-3.
only be sufficient to replace arginine lost in creatine
NOS-1 (also known as nNOS, NOS-I and Type I NOS) is
production. However, keep in mind that protein turnover
constitutive and is predominately located in neuronal tissue.
and de novo synthesis also add to plasma arginine levels.
NOS-2 (also known as iNOS, NOS-II and Type II NOS) is
Another metabolic pathway that involves arginine is the
inducible and is located in a variety of tissue. NOS-3 (also
synthesis of polyamines. Polyamines (putrescine, spermine
known as eNOS, NOS-3 and Type III) is constitutive and is
and spermidine) function in membrane transport, (reviewed
primarily localized in endothelial tissue. Both NOS-1 and
in ), cell growth, cell proliferation and cell differentia-
NOS-3 produce low levels of nitric oxide and are calcium
tion . Arginine and products of arginine metabo-
dependent. Agmatine increases activity of NOS-1 and
lism are necessary in both the regulation and the synthesis
NOS-3 by stimulating the release of calcium (reviewed in
However, agmatine indirectly decreases NOS-2 activ-
C. Nieves Jr, B. Langkamp-Henken / Biomed Pharmacother 56 (2002) 471–482
ity. Agmatine is converted to agmatine aldehyde by diamine
the control diet. Researchers have used arginine-free diets or
oxidase, in turn, agmatine aldehyde inhibits NOS-2
“standard” diets. The standard diets vary in arginine content
from 0.4 to 2% arginine. Ronnenberg et al. compared anarginine-free diet to a standard diet (1% arginine) in‘healthy’ young and aged rats . They showed an
3. Arginine and immunity
increase in concanavalin A-induced splenocyte inter-leukin-2 production with the standard diet compared withthe arginine-free diet. Supplementing arginine in the diet
The importance of arginine in metabolic pathways result-
(3% arginine) did not increase interleukin-2 production
ing in protein formation and removal of nitrogenous waste
above that seen with the standard diet . Kobayashi et al.
as well as cell signaling, proliferation and differentiation has
compared antigen-specific mucosal immune responses in
been well established. What remains to be clarified is
mice fed an arginine-free diet with verses fed an arginine-
whether dietary arginine can be supplemented at pharmaco-
supplemented diet containing 8.7 g/l of arginine Mice
logic levels to manipulate metabolic outcome. Currently,
fed the arginine-supplemented diet had a higher level of
clinicians are adding arginine to enteral formulas at a
antigen-specific fecal immunoglobulin A. Mice fed the
concentration of five times that found in a typical diet in
arginine-free and arginine-supplemented diets did not differ
attempt to boost immune function and improve clinical
in daily consumption of the diet or in body weight .
outcome in critically ill patients. The rationale for thispractice is based on a number of studies demonstrating the
While arginine may not be required in the diet of a
benefit of arginine supplementation on immune function.
healthy adult animal to maintain weight or nitrogen balance,
However, missing from critical reviews and possibly even
it may be required in the diet to maintain normal immune
the literature in general have been the studies showing no
function. In fact, some studies using lymphocytes from
effect or a detrimental effect of arginine supplementation on
healthy animals demonstrate depressed in vitro lymphocyte
immune function or outcome. Twenty-one animal studies in
proliferation when cultured in media containing low levels
which arginine was supplemented enterally are summarized
of arginine and maximal proliferation when arginine is
in The effect of arginine supplementation on
added back to the media at physiologic plasma concentra-
various immune parameters is listed as an increase, no
tions If a minimal amount of arginine is required
change, or a decrease in the immune parameter. From this
to maintain immune function, the question becomes how
table it becomes evident that the effects of arginine supple-
much arginine is enough? This question is difficult to
mentation on immune function are not consistent. For
answer due to the lack of uniformity in the amount of
example, five studies report an increase in thymus weight
arginine supplemented in the experimental diets, the large
with arginine supplementation while five studies report no
variation in arginine content of the “standard” control diets,
change in thymus weight with supplementation. Two studies
and the nitrogen source and/or content of diets.
report an increase in mitogen-induced in vitro interleukin-2
Nutritionists tend to compare arginine supplementation
production while five studies report no change in
to an isonitrogenous control diet to differentiate between the
interleukin-2 production with arginine supplementation.
non-specific effects of arginine (high nitrogen content) and
Recommendations for pharmacologic supplementation of
the specific effects of arginine and arginine metabolites.
arginine to enhance immunity in human health and patholo-
While normalizing the nitrogen load between treatment
gies cannot be made until we have a clearer picture of the
groups may not be as big of a concern in healthy animals
basis for and/or outcomes of these recommendations.
and humans, it does become an issue with stress models
Many factors contribute to the inconsistent effects of
(e.g. sepsis and trauma), which result in negative nitrogen
enteral arginine supplementation on immune function. One
of the most evident factors is the amount of arginine
Commonly, glycine, alanine, or a mixture of nonessential
supplemented in the diet. Researchers have provided 5 g of
amino acids is added to the control diet to balance the
arginine per kg diet (with half provided in the diet and half
nitrogen load between arginine-supplemented and control
provided in the drinking water) to 17 g of arginine per liter
diets. Because arginine is a nitrogen-rich amino acid,
of liquid diet. Others have provided 100 mg arginine HCl
non-physiologic concentrations of single amino acids must
via gavage (see The different concentrations of
be added to make the control diet to make it isonitrogenous.
supplemental arginine and methods of administration may
Unfortunately this is not without consequence. Pharmaco-
contribute to the inconsistent effects of arginine supplemen-
logic doses of glycine have been reported to alter free amino
tation on immunity. Additionally, these differences also
acid patterns and intestinal enzyme activity in a sepsis
make it difficult to compare studies.
model Glycine may also have cytoprotective effects
Another factor that may contribute to the inconsistent
(reviewed in While the addition of alanine for a
effects of arginine supplementation on immune function is
nitrogen control in a sepsis model appears to preserve
Table 1Summary of animal studies showing the effect of arginine administered enterally on various immune parameters and outcomes
Effect of arginine on immune parameters and outcomes
Standard a diet with added arginine (2% total
arginine) vs. a standard diet a madeisonitrogenous with added alanine for 14 d
Purified amino acid diet with 3% total arginine
vs. 1.1% total arginine (control diet) vs.
Standard diet with 1% arginine in the drinking NK activity, IL-2R expression, T DTH, IL-2 productionwater vs. standard diet with an isonitrogenous cell cytotoxicity
level of glycine in the drinking water for 10 d
Standard diet (1.8% arginine) with added 0.5,
1, 2, or 3% arginine—half provided in the diet proliferation d
and half in the drinking water vs. standard diet(1.8% arginine) for 6 d
Liquid diet with 8.7 g/l arginine vs. arginine-
free liquid diet made isonitrogenous withadded non-essential amino acids for 14–21 d
Standard diet with 1.2 vs. 0% arginine in the
Liquid diet with 7.7 g/l vs. 0 g/l total arginine
Isonitrogenous liquid diets with 2.4, 4.5, 7.2 or DTH with 7.2 g/l
12 g total arginine per l via gastrostomy for14 d
Standard diet with added 2% arginine vs.
standard diet made isonitrogenous with added
Standard diet with added 2% arginine vs.
with
E. coli , and 20% whole standard diet made isonitrogenous with added
Standard diet (1.6% arginine) with 0.75 vs. 0%
Liquid diet via gastrostomy containing 5, 11,
or 17 g/l arginine HCl vs. 0.7 g/l total arginine
Standard diet (1.8% arginine) with 100 mg
arginine HCl vs. water via gavage for 4–7 d
Standard diet plus 1, 2, or 5% arginine vs.
Splenocyte proliferation, survival Survival with 5% arginine
Effect of arginine on immune parameters and outcomes
Standard diet (2% arginine) with 1.8 vs. 0%
arginine in the drinking water (0% madeisonitrogenous with added glycine) for 21 d
Standard diet with added arginine (3% total
arginine) vs. standard diet (1.6% arginine)
made isonitrogenous with added alanine for
Liquid diet with 2.93 g/l total arginine vs.
arginine-free liquid diet made isonitrogenous
bacterial killing, splenocyteproliferation, IL-2 production
Standard diet (1.8% arginine) with added 1%
arginine—half provided in the diet and half in proliferation
the drinking water vs. standard diet (1.8%arginine) for 6 d
Standard diet plus 49 g/kg arginine vs.
standard diet made isonitrogenous with added
Chemical-induced colorectal Standard diet with 1 vs. 0% arginine in the
Thymus weight after 10 week of Thymus weight after 22 week of Tumor incidence, burden, and
Standard diet with 1% arginine in drinking
water vs. standard diet with an isonitrogenous IL-2 production, splenocytelevel of glycine in the drinking water for
a Unless otherwise noted, standard diets contain approximately 0.4 to 0.98% arginine.
b Abbreviations: DTH, delayed-type hypersensitivity; IgA, immunoglobulin A; IL-1 , interleukin-1 beta; IL-2R, interleuking-2 receptor; mRNA, messenger RNA; NK, natural killer; and TNF-α,
c In vitro production of IL-2 from mitogen-stimulated lymphocyte cultures.
d Unless stated otherwise splenocyte and thymocyte proliferation is mitogen induced.
C. Nieves Jr, B. Langkamp-Henken / Biomed Pharmacother 56 (2002) 471–482
plasma and tissue-free amino acid concentrations
In addition to differences in lymphocyte cytokine pro-
observations from our laboratory suggest that alanine is not
duction between different strains of mice, macrophages
from different strains of mice respond differently. Resident
In 2000, we showed that arginine supplementation en-
peritoneal macrophages from C57BL/6 are more easily
hanced delayed-type hypersensitivity responses in young,
activated to produce nitric oxide upon stimulation with
adult, and aged male CB6F1 (BALB/c √ó C57BL/6) mice
interferon-γ than macrophages from BALB/c mice
given a 2% arginine diet versus an isonitrogenous control
Based on differences in lymphocyte cytokine production
diet The control diet was made isonitrogenous with the
and macrophage activation among strains of mice, it may be
addition of alanine. Recently we have repeated this experi-
possible that arginine supplementation yields different im-
ment in female BALBc mice, but this time a third diet was
mune outcomes in different strains of mice. Similar obser-
added to the protocol. Mice received the standard diet with
vations may also become apparent between species with
added arginine (2% total arginine), the standard diet made
arginine supplementation (and reviewed in ).
isonitrogenous to the arginine diet with the addition of
Up until this point in the review, the experimental model
alanine, or the standard diet. Delayed-type hypersensitivity
used to examine the effect of arginine supplementation on
responses were not different between mice receiving the 2%
immune function has been largely ignored. The amount of
arginine diet and the mice that received the standard diet.
arginine required in the diet and ultimately the effect of
However, the delayed-type hypersensitivity response was
arginine supplementation on outcome might largely depend
significantly lower in the mice fed the alanine-supplemented
on the experimental model. Sepsis and inflammation in-
(isonitrogenous diet) than the standard diet (unpublished
crease nitric oxide production via upregulation of NOS-2
data). These data in conjunction with the previously re-
Mice that lack NOS-2 are susceptible to infection
ported data suggest that arginine supplementation did
Trauma, on the other hand, is associated with
not enhance delayed-type hypersensitivity responses but
decreased nitric oxide production and increased extrahe-
that the addition of alanine to the standard diet depressed
patic arginase-I expression and activity
these responses. Other explanations for these data could be
The greatest increase in extrahepatic arginase activity is
that male and female mice or different strains of mice
found in splenic macrophages . In humans, general
respond differently to arginine supplementation. The latter
surgery and trauma increase peripheral mononuclear cell
explanation could also help justify some of the conflicting
arginase-I activity and decrease plasma nitric oxide
effects of arginine supplementation on immune function that
In general surgery patients, the increase in mononuclear
arginase activity was only evident when the T 2 cytokine,
Mitogen-stimulated splenocytes from different strains of
interleukin 10, was increased Bernard et al. demon-
mice have a propensity to secrete different cytokines. For
strated that in vitro macrophage arginase activity increases
example, splenocytes from C57BL/6 mice secrete higher
concentrations of interferon-γ and lower concentrations of
-adrenoceptor blockade prior to trauma decreases murine
interleukin-4, while splenocytes from BALB/c mice secrete
splenic arginase activity These data suggest a role for
lower concentrations of interferon-γ and higher concentra-
catecholamines and cytokines in switching arginine metabo-
tions of interleukin-4 Helper T lymphocytes that
lism between an antimicrobial and a tissue repair pathway.
Since arginase and nitric oxide synthase utilize arginine
interleukin-2 and tumor-necrosis factor- are referred to as
as a substrate, increased expression of arginase-I increases
T helper-1 (T 1) cells, whereas T lymphocytes that pre-
intracellular ornithine and polyamines and reduces basal
dominantly produce interleukin-4, -5, -6 and -10 are re-
nitric oxide synthesis by endothelial cells . Nitric oxide
ferred to as T 2 cells. Cell-mediated immune functions,
derived from NOS-3 produces vasorelaxation and inhibits
such as delayed-type hypersensitivity and activation of
platelet aggregation and neutrophil infiltration—important
cytotoxic T lymphocytes and inflammatory macrophages,
functions for maintaining organ blood flow following
are associated with T 1 responses, while B lymphocyte,
trauma A series of studies examined whether
mast cell and eosinophil activation are associated with T 2
intravenous arginine administration during resuscitation
responses. The balance between T 1 and T 2 responses
improved blood flow to various organs following trauma
predicts the metabolic fate of arginine and possibly the
and hemorrhage . Rats underwent a sham operation or
difference between cell proliferation and connective tissue
laparotomy (trauma), were bled to a mean arterial pressure
production or antimicrobial capacity. In vitro and in vivo
of 40 mmHg, and resuscitated. Arginine (300 mg/kg) or
studies demonstrate that NOS-2 and arginase-1 are differ-
saline was infused intravenously during the first 15 min of
entially regulated by T 1- and T 2-driven immune reac-
resuscitation. At 3 h post trauma-hemorrhage and resusci-
tions with T 1 cytokines inducing NOS-2 and T 2 cytok-
tation, plasma arginine (corrected for the plasma protein
concentration) decreased 87% compared with shamoperated
C. Nieves Jr, B. Langkamp-Henken / Biomed Pharmacother 56 (2002) 471–482
group. Infusion of arginine during resuscitation temporarily
Two of the trials show an increase in mitogen-induced
corrected plasma arginine concentrations and significantly
lymphocyte proliferation, while three of the trials show no
increased corrected plasma citrulline concentrations
increase or a decrease in lymphocyte proliferation with
Cardiac output and blood flow to the heart, skin, muscle,
arginine supplementation. No studies demonstrate improved
kidneys, stomach, pancreas, small intestine, large intestine,
clinical outcomes with arginine supplementation.
mesentery, brain, liver, and spleen were increased with
A large part of the rationale for supplementing arginine to
intravenous arginine administration during resuscitation
enhance immune function is based on studies that show an
. Arginine infusion during resuscitation was also asso-
increase in mitogen-induced in vitro lymphocyte prolifera-
ciated with decreased liver injury and plasma interleukin-6
tion (see In vitro lymphocyte proliferation
and increased splenocyte interleukin-2 production com-
is a convenient measure of immune function, especially in
pared with animals resuscitated without arginine
human studies, and appears to correlate with mortality
Mitogen-induced splenocyte proliferation was significantly
However, the mechanism by which arginine supplementa-
lower following trauma-hemorrhage and resuscitation with-
tion in vivo may enhance in vitro lymphocyte proliferation
out arginine infusion compared with sham-operated con-
is still unknown. Is it possible that mitogen-induced in vitro
trols. Arginine infusion prevented this decrease Based
lymphocyte proliferation represents a completely artificial
on the kinetics of nitric oxide produc tion in the blood flow
condition? For example, Barbul et al. gave healthy volun-
studies, the authors concluded that the intravenous arginine
teers 24.8 g of free arginine per day for 14 d . Plasma
increased blood flow via the production of nitric oxide by
arginine concentrations were 66.3 µM at baseline and in-
creased to 114 µM after 14 d of supplementation. Peripheral
While this series of studies showed benefit of intravenous
blood lymphocytes were obtained at baseline and day 14
infusion of arginine at the time of resuscitation, it is
and stimulated with mitogen in culture media (RPMI 1640)
unknown and perhaps doubtful that enteral arginine would
containing approximately 1150 µM arginine Any
have the same affect. In a healthy animal, approximately
changes that occur in vivo due to the difference in plasma
40% of dietary arginine is degraded in the intestine in the
arginine concentrations may be abolished in vitro. Ochoa et
al. demonstrated that interleukin-2 accumulation was sig-
hemorrhage, blood flow to the intestines and portal blood
nificantly less when splenocytes, stimulated with anti-CD3,
flow are reduced which may alter the absorption process
were cultured in media containing 100 or 1000 µM arginine
Madden et al. demonstrated that providing arginine
compared with cells cultured with 0 or 10 µM arginine
(100 mg arginine HCl every 12 h) via gavage immediately
Additionally, CD45RA negative CD8+ (memory) T cells
after and for 4 d following cecal ligation and puncture that
and the number of CD8 and CD3 receptors were upregu-
overall survival was not altered compared with unsupple-
lated with the addition of arginine to the culture media
mented controls When the arginine was gavaged daily
Rodriguez et al. demonstrated that T cell receptor CD3
for 3 d prior to and after the insult following the same
chain expression was regulated by arginine in the culture
administration regimen, survival improved significantly.
Beneficial effects on survival were also observed when the
signal transduction element and the rate-limiting step in the
arginine was infused intravenously immediately after insult.
assembly and membrane expression of the T cell receptor
These data suggest that enteral arginine supplementation
prior to injury or intravenous arginine supplementation at
not interleukin-2 production or interleukin-2 receptor
the time of injury may provide the most benefit . Thus
chains) was reduced when Jurkat cells (helper T-cell line)
timing of enteral arginine supplementation appears to be
were cultured in arginine-free media. The inhibition of the
another factor contributing to the inconsistent outcomes
chain expression was completely reversed with the addition
associated with arginine supplementation.
of arginine to the culture media This arginine-mediated
The strongest evidence supporting the role of arginine
effect was attributed to a change in CD3 mRNA half-life
supplementation on immune-modulation in humans comes
from double-blind, randomized controlled trials. However,
Changes in macrophage function with altered cell culture
there are few such trials and fewer that investigate or
arginine concentrations have also been described. Albina et
demonstrate clinical outcomes (this does not include clinical
al. demonstrated that macrophage superoxide production,
trials that compare immune-enhancing enteral formulas of
phagocytosis, protein synthesis, and tumoricidal activity
which arginine is one of many added nutrients). Of 15
were greatest in culture media containing 6 µM arginine,
clinical trials that examined the effect of arginine supple-
decreased as the arginine concentration in the media ap-
proached physiologic concentrations (∼100 µM), and con-
tinued to decrease or maintained lower levels of function at
the study was a double-blind, randomized controlled trial.
pharmacologic concentrations observed in common cell
C. Nieves Jr, B. Langkamp-Henken / Biomed Pharmacother 56 (2002) 471–482
Table 2Double-blind, randomized controlled trials investigating the effect of enteral arginine supplementation on immune function
No differences in lymphocyte No difference in
preoperatively via oral intake proliferation or endotoxin-
lymphocyte proliferation and complications
20, 10, or 0 g/d for 4 weeks, No change in viral load and
17, 8.5, or 0 g/d for 4 weeks No change to possibly a in
residents with pressure ulcers via oral intake
lymphocyte proliferation andno change in IL-2 production
a Abbreviations: IL-2, interleukin-2; GI, gastrointestinal.
culture media (∼1200 µM) These data suggest that
arginine supplementation, the animal species or strain of
macrophage function would be enhanced at low plasma
species, and the experimental model. Systematic study is
arginine concentrations and inhibited at concentrations typi-
required to determine whether a basal dietary intake of
cally found in plasma or culture media. While there are very
arginine is required to maintain immune function during
few in vivo measures of immune function that can be used
health and how much arginine is required to meet metabolic
to assess the immune effects of arginine supplementation
requirements during periods of growth or stress.
(especially in humans), caution should be used wheninterpreting in vitro studies with immune cells cultured inmedia containing nonphysiologic concentrations of argin-
Acknowledgments
In summary, arginine functions in the body as a free
This research was supported by the Florida Agricultural
amino acid, a component of most proteins, and the substrate
Experiment Station, and approved for publication as Journal
for several non-protein, nitrogen-containing compounds,
many of which function in immunity. Although arginine issynthesized in the body, it is not made in sufficient quanti-ties to support growth or meet metabolic requirements
References
during periods of stress. Based on the biochemical andphysiological role of arginine in maintaining health and
Albina JE, Caldwell MD, Henry Jr WL, Mills CD. Regulation of
immunity, arginine is being added at pharmacologic con-
macrophage functions by L-arginine. J Exp Med 1989;169:1021–9.
centrations to enteral formulas to boost immune function.
Albina JE, Henry Jr L. Suppression of lymphocyte proliferation
Unfortunately, animal and human studies that investigate
through the nitric oxide synthesizing pathway. J Surg Res 1991;50:
enteral arginine supplementation as the single variable do
not show clear immunologic benefit. The inconsistent ef-
Alheid U, Frolich JC, Forstermann U. Endothelium-derived relaxing
fects of arginine supplementation on immune function are
factor from cultured human endothelial cells inhibits aggregation of
due to numerous factors, such as the amount and timing of
human platelets. Thromb Res 1987;47:561–71.
C. Nieves Jr, B. Langkamp-Henken / Biomed Pharmacother 56 (2002) 471–482
Angele MK, Fitzal F, Smail N, Knoferl MW, Schwacha MG,
Cui X, Iwasa M, Iwasa Y, Ogoshi S. Arginine-supplemented diet
Ayala A, et al. L-Arginine attenuates trauma-hemorrhage-induced
decreases expression of inflammatory cytokines and improves sur-
liver injury. Crit Care Med 2000;28:3242–8.
vival in burned rats. JPEN J Parenter Enteral Nutr 2000;24:89–96.
Angele MK, Smail N, Knoferl MW, Ayala A, Cioffi WG,
Daly JM, Reynolds J, Thom A, Kinsley L, Dietrick-Gallagher M,
Chaudry IH. L-Arginine restores splenocyte functions after trauma
Shou J, et al. Immune and metabolic effects of arginine in the
and hemorrhage potentially by improving splenic blood flow. Am J
surgical patient. Ann Surg 1988;208:512–23.
Elitsur Y, Strom J, Luk GD. Inhibition of ornithine decarboxylase
Angele MK, Smail N, Wang P, Cioffi WG, Bland KI, Chaudry IH.
activity decreases polyamines and suppresses DNA synthesis in
L-Arginine restores the depressed cardiac output and regional
human colonic lamina propria lymphocytes. Immunopharmacology
perfusion after trauma-hemorrhage. Surgery 1998;124:394–401
Fong WF, Heller JS, Canellakis ES. The appearance of an ornithine
Babal P, Ruchko M, Campbell CC, Gilmour SP, Mitchell JL,
decarboxylase inhibitory protein upon the addition of putrescine to
Olson JW, et al. Regulation of ornithine decarboxylase activity and
cell cultures. Biochim Biophys Acta 1976;428:456–65.
polyamine transport by agmatine in rat pulmonary artery endothelial
Gennari R, Alexander JW. Arginine, glutamine, and dehydroepi-
cells. J Pharmacol Exp Ther 2001;296:372–7.
androsterone reverse the immunosuppressive effect of prednisoneduring gut-derived sepsis. Crit Care Med 1997;25:1207–14.
Baligan M, Giardina A, Giovannini G, Laghi MG, Ambrosioni G.
Gianotti L, Alexander JW, Pyles T, Fukushima R. Arginine-
L-Arginine and immunity. Study of pediatric subjects. Minerva
supplemented diets improve survival in gut-derived sepsis and
peritonitis by modulating bacterial clearance. The role of nitric
Barbul A, Lazarou SA, Efron DT, Wasserkrug HL, Efron G.
oxide. Ann Surg 1993;217:644–53 discussion 653-654.
Arginine enhances wound healing and lymphocyte immune
Gobert AP, Daulouede S, Lepoivre M, Boucher JL, Bouteille B,
responses in humans. Surgery 1990;108:331–6 [discussion 336–7].
Buguet A, et al. L-Arginine availability modulates local nitric oxide
Barbul A, Sisto DA, Wasserkrug HL, Efron G. Arginine stimulates
production and parasite killing in experimental trypanosomiasis.
lymphocyte immune response in healthy human beings. Surgery
Gonce SJ, Peck MD, Alexander JW, Miskell PW. Arginine supple-
Barbul A, Wasserkrug HL, Seifter E, Rettura G, Levenson SM,
mentation and its effect on established peritonitis in guinea pigs.
Efron G. Immunostimulatory effects of arginine in normal and
JPEN J Parenter Enteral Nutr 1990;14:237–44.
injured rats. J Surg Res 1980;29:228–35.
Hall JC. Glycine. JPEN J Parenter Enter Nutr 1998;22:393–8.
Barbul A, Wasserkrug HL, Sisto DA, Seifter E, Rettura G, Leven-
Hayashi S, Murakami Y, Matsufuji S. Ornithine decarboxylase
son SM, Efron G. Thymic stimulatory actions of arginine. JPEN J
antizyme: a novel type of regulatory protein. Trends Biochem Sci
Parenter Enteral Nutr 1980;4:446–9.
Bauer H, Jung T, Tsikas D, Stichtenoth DO, Frolich JC, Neumann C.
Hesse M, Modolell M, La Flamme AC, Schito M, Fuentes JM,
Nitric oxide inhibits the secretion of T-helper 1- and T-helper
Cheever AW, et al. Differential regulation of nitric oxide synthase-2
2-associated cytokines in activated human T cells. Immunology
and arginase-1 by type 1/type 2 cytokines in vivo: granulomatous
pathology is shaped by the pattern of L-arginine metabolism. J
Bermudez LE. Differential mechanisms of intracellular killing of
Mycobacterium avium and
Listeria monocytogenes by activated
Heys SD, Segar A, Payne S, Bruce DM, Kernohan N, Eremin O.
human and murine macrophages. The role of nitric oxide. Clin Exp
Dietary supplementation with L-arginine: modulation of tumour-
infiltrating lymphocytes in patients with colorectal cancer. Br J Surg
Bernard AC, Fitzpatrick EA, Maley ME, Gellin GL, Tsuei BJ,
Arden WA, et al. Beta adrenoceptor regulation of macrophage
Iyer RK, Kim HK, Tsoa RW, Grody WW, Cederbaum SD. Cloning
arginase activity. Surgery 2000;127:412–8.
and characterization of human agmatinase. Mol Genet Metab
Bernard AC, Mistry SK, Morris Jr SM, O’Brien WE, Tsuei BJ,
Maley ME, et al. Alterations in arginine metabolic enzymes in
Kennedy JA, Kirk SJ, McCrory DC, Halliday MI, Barclay GR,
Rowlands BJ. Modulation of immune function and weight loss byL-arginine in obstructive jaundice in the rat. Br J Surg 1994;81:
Blantz RC, Satriano J, Gabbai F, Kelly C. Biological effects of
arginine metabolites. Acta Physiol Scand 2000;168:21–5.
Kirk SJ, Hurson M, Regan MC, Holt DR, Wasserkrug HL, Barbul A.
Borman A, Wood TR, Black HC, Anderson EG, Oesterling MJ,
Arginine stimulates wound healing and immune function in elderly
Wommack E, et al. The role of arginine in growth with some
human beings. Surgery 1993;114:155–9 [discussion 160].
observations on the effects of argininic acid. J Biol Chem 1946;166:
Kirk SJ, Regan MC, Wasserkrug HL, Sodeyama M, Barbul A.
Arginine enhance T-cell responses in athymic nude mice. JPEN
Brittenden J, Park KG, Heys SD, Ross C, Ashby J, Ah-See A, et al.
Parenter Enteral Nutr 1992;16:429–32.
L-Arginine stimulates host defenses in patients with breast cancer.
Kles KA, Wallig MA, Tappenden KA. Luminal nutrients exacerbate
intestinal hypoxia in the hypoperfused jejunum. JPEN J Parenter
Castillo L, Chapman TE, Yu YM, Ajami A, Burke JF, Young VR.
Dietary arginine uptake by the splanchnic region in adult humans.
Knopf RF, Conn JW, Fajans SS, Floyd JC, Guntsche EM, Rull JA.
Plasma growth hormone response to intravenous administration of
Chambon-Savanovitch C, Felgines C, Farges MC, Raul F,
amino acids. J Clin Endocrinol Metab 1965;25:1140–4.
Cezard JP, Davot P, et al. Comparative study of glycine, alanine or
Kobayashi T, Yamamoto M, Hiroi T, McGhee J, Takeshita Y,
casein as inert nitrogen sources in endotoxemic rats. J Nutr 1999;
Kiyono H. Arginine enhances induction of T helper 1 and T helper
2 cytokine synthesis by Peyer’s patch alpha beta T cells and
Cockcroft DW, Gault MH. Prediction of creatinine clearance from
antigen-specific mucosal immune response. Biosci Biotechnol Bio-
serum creatinine. Nephron 1976;16:31–41.
C. Nieves Jr, B. Langkamp-Henken / Biomed Pharmacother 56 (2002) 471–482
Kubes P, Kanwar S, Niu XF, Gaboury JP. Nitric oxide synthesis
Murasko DM, Weiner P, Kaye D. Association of lack of mitogen-
inhibition induces leukocyte adhesion via superoxide and mast cells.
induced lymphocyte proliferation with increased mortality in the
elderly. Aging Immunol Infect Dis 1988;1:1–6.
Langkamp-Henken B, Herrlinger-Garcia KA, Stechmiller JK, Nick-
Nakagawa I, Kobayashi K, Suzuki T, Takahashi T. Amino acid
erson-Troy JA, Lewis B, Moffatt L. Arginine supplementation is
requirements of children—minimal needs of tryptophan, arginine
well tolerated but does not enhance mitogen-induced lymphocyte
and histidine based on nitrogen balance method. J Nutr 1963;80:
proliferation in elderly nursing home residents with pressure ulcers.
JPEN J Parenter Enter Nutr 2000;24:280–7.
Nirgiotis JG, Hennessey PJ, Andrassy RJ. The effects of an
Langkamp-Henken B, Johnson LR, Viar MJ, Geller AM, Kotb M.
arginine-free enteral diet on wound healing and immune function in
Differential effect on polyamine metabolism in mitogen- and
the postsurgical rat. J Pediatr Surg 1991;26:936–41.
superantigen-activated human T-cells. Biochim Biophys Acta1
Nussler AK, Billiar TR. Inflammation, immunoregulation, and
inducible nitric oxide synthase. J Leukocyte Biol 1993;54:171–8.
Laursen JB, Boesgaard S, Trautner S, Rubin I, Poulsen HE,
Ochoa JB, Bernard AC, Mistry SK, Morris Jr SM, Figert PL,
Aldershvile J. Endothelium-dependent vasorelaxation in inhibited
Maley ME, et al. Trauma increases extrahepatic arginase activity.
by in vivo depletion of vascular thiol levels: role of endothelial nitric
oxide synthase. Free Radic Res 2001;35:387–94.
Ochoa JB, Bernard AC, O’Brien WE, Griffen MM, Maley ME,
Lewis B, Langkamp-Henken B. Arginine enhances in vivo immune
Rockich AK, et al. Arginase I expression and activity in human
responses in young, adult and aged mice. J Nutr 2000;130:1827–30.
mononuclear cells after injury. Ann Surg 2001;233:393–9.
Li H, Meininger CJ, Hawker Jr JR, Haynes TE, Kepka-Lenhart D,
Ochoa JB, Strange J, Kearney P, Gellin G, Endean E, Fitzpatrick E.
Mistry SK, et al. Regulatory role of arginase I and II in nitric oxide,
Effects of L-arginine on the proliferation of T lymphocyte subpopu-
polyamine, and proline syntheses in endothelial cells. Am J Physiol
lations. JPEN J Parenter Enter Nutr 2001;25:23–9.
Endocrinol Metab 2001;280(E):75–82.
Ochoa JB, Udekwu AO, Billiar TR, Curran RD, Cerra FB, Sim-
Ma Q, Hoper M, Anderson N, Rowlands BJ. Effect of supplemental
mons RL, et al. Nitrogen oxide levels in patients after trauma and
L-arginine in a chemical-induced model of colorectal cancer. World
during sepsis. Ann Surg 1991;214:621–6.
Pacelli R, Wink DA, Cook JA, Krishna MC, DeGraff W, Fried-
Madden HP, Breslin RJ, Wasserkrug HL, Efron G, Barbul A.
man N, et al. Nitric oxide potentiates hydrogen peroxide-induced
Stimulation of T cell immunity by arginine enhances survival in
killing of
Escherichia coli . J Exp Med 1995;182:1469–79.
peritonitis. J Surg Res 1988;44:658–63.
Park KG, Hayes PD, Garlick PJ, Sewell H, Eremin O. Stimulationof lymphocyte natural cytotoxicity by L-arginine. Lancet 1991;337:
McCann PP, Tardif C, Mamont PS. Regulation of ornithine decar-
boxylase by ODC-antizyme in HTC cells. Biochem Biophys ResCommun 1977;75:948–54.
Park KG, Heys SD, Blessing K, Kelly P, McNurlan MA,Eremin O, et al. Stimulation of human breast cancers by dietary
McCarter MD, Gentilini OD, Gomez ME, Daly JM. Preoperative
L-arginine. Clin Sci (Colch) 1992;82:413–7.
oral supplement with immunonutrients in cancer patients. JPEN J
Peck MD, Babcock GF, Alexander JW, Billiar T, Ochoa J. High
Parenter Enter Nutr 1998;22:206–11.
doses of dietary arginine during repletion impair weight gain and
Merimee TJ, Lillicrap DA, Rabinowitz D. Effect of arginine on
increase infectious mortality in protein-malnourished mice. Br J
serum-levels of human growth-hormone. Lancet 1965;2:668–70.
Merimee TJ, Rabinowitz D, Riggs L, Burgess JA, Rimoin DL,
Regunathan S, Reis DJ. Characterization of arginine decarboxylase
McKusick VA. Plasma growth hormone after arginine infusion.
in rat brain and liver: distinction from ornithine decarboxylase. J
Clinical experiences. New Engl J Med 1967;276:434–9.
Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-M-2
Reynolds JV, Daly JM, Shou J, Sigal R, Ziegler MM, Naji M.
macrophages and the Th1/Th2 paradigm. J Immunol 2000;164:
Immunologic effects of arginine supplementation in tumor-bearing
and non-tumor-bearing hosts. Ann Surg 1990;211:202–10.
Minami Y, Weissman AM, Samelson LE, Klausner RD. Building a
Reynolds JV, Daly JM, Zhang S, Evantash E, Shou J, Sigal R,
multichain receptor: synthesis, degradation, and assembly of the
Ziegler MM. Immunomodulatory mechanisms of arginine. Surgery
T-cell antigen receptor. Proc Natl Acad Sci USA 1987;84:2688–92.
Morris DR. A new perspective on ornithine decarboxylase
Robinson LE, Bussiere FI, Le Boucher J, Farges MC, Cynober LA,
regulation: prevention of polyamine toxicity is the overriding theme.
Field CJ, Baracos VE. Amino acid nutrition and immune function in
tumour-bearing rats: a comparison of glutamine-, arginine- and
Morris Jr SM, Bhamidipati D, Kepka-Lenhart D. Human type II
ornithine 2-oxoglutarate- supplemented diets. Clin Sci (Lond) 1999;
arginase: sequence analysis and tissue-specific expression. Gene
Rodriguez PC, Zea AH, Culotta KS, Zabaleta J, Ochoa JB,
Munder M, Eichmann K, Modolell M. Alternative metabolic states
Ochoa AC. Regulation of T cell receptor CD3zeta chain expression
by L-arginine. J Biol Chem 2002;277:21123–9.
synthase/arginase balance: competitive regulation by CD4+ T cells
Ronnenberg AG, Gross KL, Hartman WJ, Meydani SN, Prior RL.
correlates with Th1/Th2 phenotype. J Immunol 1998;160:5347–54.
Dietary arginine supplementation does not enhance lymphocyte
Munder M, Eichmann K, Moran JM, Centeno F, Soler G, Mod-
proliferation or interleukin-2 production in young and aged rats. J
olell M. Th1/Th2-regulated expression of arginase isoforms in
murine macrophages and dendritic cells. J Immunol 1999;163:
Rose WC. The Nutritive significance of the amino acids and certain
related compounds. Science 1937;86:298–300.
Murakami Y, Matsufuji S, Miyazaki Y, Hayashi S. Destabilization of
Rose WC, Haines WJ, Warner DT. The amino acid requirements of
ornithine decarboxylase by transfected antizyme gene expression in
man V. The role of lysine, arginine, and tryptophan. J Biol Chem
hepatoma tissue culture cells. J Biol Chem 1992;267:13138–41.
C. Nieves Jr, B. Langkamp-Henken / Biomed Pharmacother 56 (2002) 471–482
Rose WC, Rice EE. The significance of the amino acids in canine
Torre PM, Ronnenberg AG, Hartman WJ, Prior RL. Oral arginine
supplementation does not affect lymphocyte proliferation during
Saito H, Trocki O, Wang SL, Gonce SJ, Joffe SN, Alexander JW.
endotoxin-induced inflammation in rats. J Nutr 1993;123:481–8.
Metabolic and immune effects of dietary arginine supplementation
Torre PM, Ronnenberg AG, Hartman WJ, Prior RL. Supplemental
after burn. Arch Surg 1987;122:784–9.
arginine and ornithine do not affect splenocyte proliferation in
Sastre M, Galea E, Feinstein D, Reis DJ, Regunathan S. Metabolism
surgically treated rats. JPEN J Parenter Enteral Nutr 1993;17:532–6.
of agmatine in macrophages: modulation by lipopolysaccharide and
Torring N, Vinter-Jensen L, Pedersen SB, Sorensen FB, Flyvbjerg A,
inhibitory cytokines. Biochem J 1998;330:1405–9.
Nexo E. Systemic administration of insulin-like growth factor I
Sastre M, Regunathan S, Galea E, Reis DJ. Agmatinase activity in
(IGF-I) causes growth of the rat prostate. J Urol 1997;158:222–7.
rat brain: a metabolic pathway for the degradation of agmatine. J
Tsuei BJ, Bernard AC, Shane MD, Shirley LA, Maley ME, Bou-
langer BR, et al. Surgery induces human mononuclear cell arginase
Satriano J, Matsufuji S, Murakami Y, Lortie MJ, Schwartz D,
I expression. J Trauma 2001;51:497–502.
Kelly CJ, et al. Agmatine suppresses proliferation by frameshiftinduction of antizyme and attenuation of cellular polyamine levels.
van Bokhorst-De Van Der Schueren MA, Quak JJ, von Blomberg-
van der Flier BM, Kuik DJ, Langendoen SI, Snow GB, et al. Effect
Satriano J, Schwartz D, Ishizuka S, Lortie MJ, Thomson SC,
of perioperative nutrition, with and without arginine supplementa-
Gabbai F, et al. Suppression of inducible nitric oxide generation by
tion, on nutritional status, immune function, postoperative morbid-
agmatine aldehyde: beneficial effects in sepsis. J Cell Physiol
ity, and survival in severely malnourished head and neck cancer
patients. Am J Clin Nutr 2001;73:323–32.
Schaefer EL, Seidenfeld J. Effects of polyamine depletion on serum
[100] Visek WJ. Arginine needs, physiological state and usual diets. A
stimulation of quiescent 3T3 murine fibroblast cells. J Cell Physiol
reevaluation. J Nutr 1986;116:36–46.
[101] Wei XQ, Charles IG, Smith A, Ure J, Feng GJ, Huang FP, et al.
Schuber F. Influence of polyamines on membrane functions. Bio-
Altered immune responses in mice lacking inducible nitric oxide
Scull CW, Rose WCI. The relation of the arginine content of the diet
[102] Weissman AM, Ross P, Luong ET, Garcia-Morales P, Jelachich ML,
to the increments in tissue arginine during growth. J Biol Chem
Biddison WE, et al. Tyrosine phosphorylation of the human T cell
antigen receptor zeta-chain: activation via CD3 but not CD2. J
Shi O, Kepka-Lenhart D, Morris Jr SM, O’Brien WE. Structure of
the murine arginase II gene. Mamm Genome 1998;9:822–4.
[103] Wiesinger H. Arginine metabolism and the synthesis of nitric oxide
Snyderman SE, Boyer A, Holt Jr LE. The arginine requirement of
in the nervous system. Prog Neurobiol 2001;64:365–91.
the infant. AMA J Dis Child 1959;92:192–5.
[104] Windmueller HG, Spaeth AE. Metabolism of absorbed aspartate,
Stechmiller JK, Lentz-Slick A, Bender BS, Hoffinger R, Langkamp-
asparagine, and arginine by rat small intestine in vivo. Arch
Henken B. Arginine versus protein supplementation in HIV-infected
men. Nutr Clin Pract 2001;16:158–64.
Swanson B, Keithley JK, Zeller JM, Sha BE. A pilot study of the
[105] Wu CW, Chi CW, Chiu CC, Wu HS, Liu WY, P’Eng FK, et al. Can
safety and efficacy of supplemental arginine to enhance immune
daily dietary arginine supplement affect the function and subpopu-
function in persons with HIV/AIDS. Nutrition 2002;18:688–90.
lation of lymphocytes in patients with advanced gastric cancer?
Taheri F, Ochoa JB, Faghiri Z, Culotta K, Park HJ, Lan MS, et al.
L-Arginine regulates the expression of the T-cell receptor zeta chain
[106] Wu G, Morris Jr SM. Arginine metabolism: nitric oxide and beyond.
(CD3zeta) in Jurkat cells. Clin Cancer Res 2001;7:958s–65s.
Source: http://belightestarbem.com.br/arquivos/1176932687_07%20-%20Arginine%20and%20immunity.pdf
Appel to Wou-Ki: International Art Flexes its Strength at Heffel’s October Online Auction William Scott ~ Still Life with Jug Thursday afternoon brought a flurry of energized bidding that resulted in an exciting close to Heffel’s October online auction of International art. Heffel is proud to announce that the sale totalled $647,000 (all prices are in Canadian dollars and include a
(Affix patient identification label here) Beneficence and Nonmaleficence Neurosurgeon and Spine Surgeon Lumbar Decompression and Pedicle Screw Fusion A. Interpreter / cultural needs ‚Ä¢ Bladder or bowel problems due to nerve root injury. This may be temporary or permanent. ‚Ä¢ Injury to the covering of the spinal cord. This may If yes , is a qualified Interpreter present? ‚Ä
A |
B |
C |
D |
E |
F |
G |
H |
I |
J |
K |
L |
M |
N |
O |
P |
Q |
R |
S |
T |
U |
V |
W |
X |
Y |
Z |
0-9 |

 Dossier: Free amino acids in human health and pathologies
Arginine and immunity: a unique perspective
Carmelo Nieves Jr, Bobbi Langkamp-Henken *
Food Science and Human Nutrition Department, University of Florida, PO Box 110370, Gainesville, FL 32611-0370, USA
Abstract
Dossier: Free amino acids in human health and pathologies
Arginine and immunity: a unique perspective
Carmelo Nieves Jr, Bobbi Langkamp-Henken *
Food Science and Human Nutrition Department, University of Florida, PO Box 110370, Gainesville, FL 32611-0370, USA
Abstract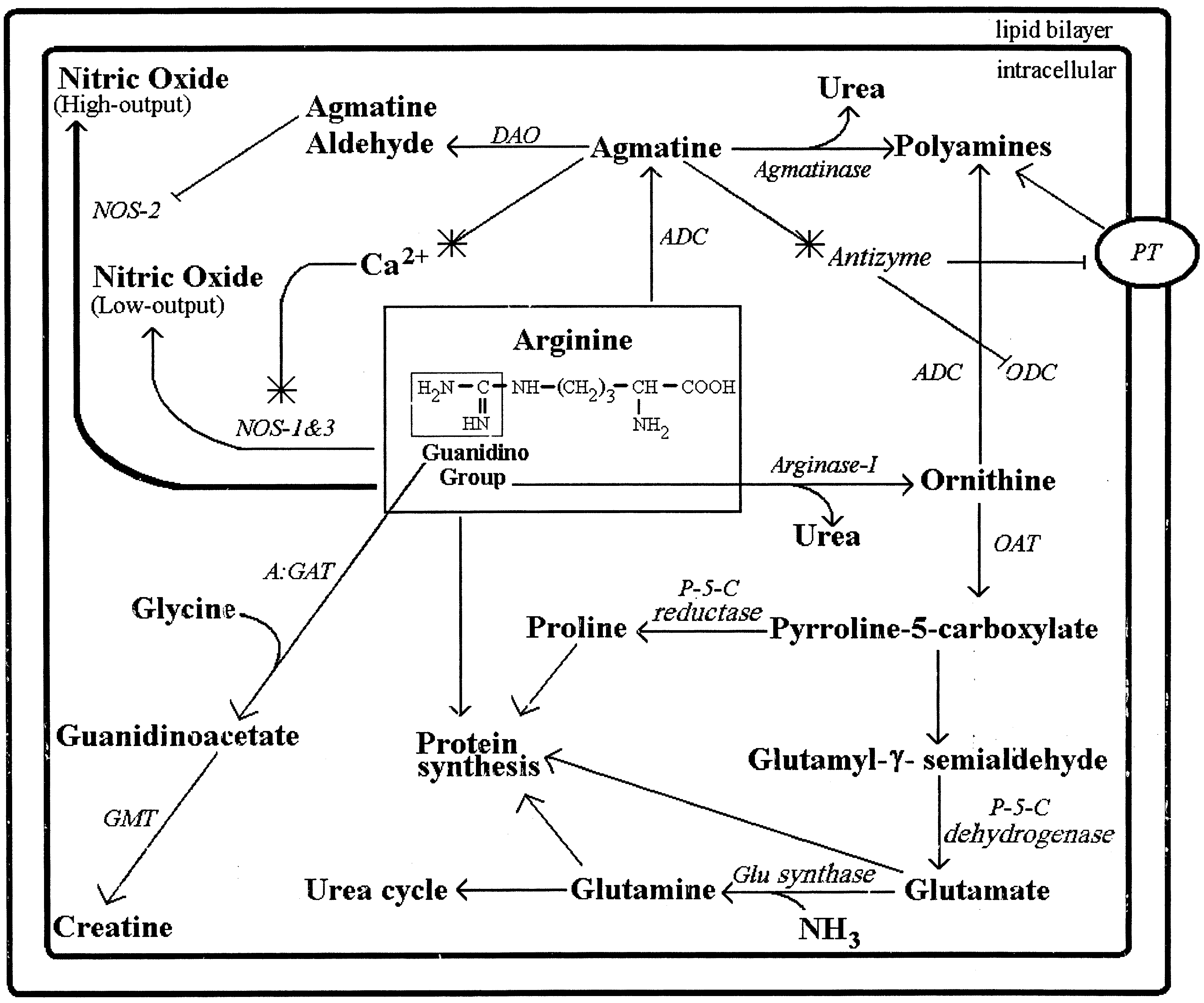

 C. Nieves Jr, B. Langkamp-Henken / Biomed Pharmacother 56 (2002) 471–482
This is another ‘condition’ where arginine may become
virtually all tissues including the kidney, brain, liver, skel-
etal muscle and lungs However, the purpose of this
One of the major functions of arginine within the body is
as an intermediate in the urea cycle. The free amino acid
Another function of arginine is protein synthesis. Argin-
arginine contains a guanidino group, which is essential for
ine can be converted into proline, glutamate and glutamine
the synthesis of urea in most mammals. Urea is primarily
(see all of which are common amino acids found
synthesized in the liver and excreted by the kidneys. In the
within most proteins. Synthesis of these three amino acids
cytosol of hepatocytes, arginase-I removes the guanidino
begins with arginine being converted into ornithine. Orni-
group from arginine to produce urea and ornithine (see
thine is then converted into pyrroline-5-carboxylate. The
Urea is then transported from the hepatocyte into the
enzyme for this reaction is ornithine aminotransferase.
C. Nieves Jr, B. Langkamp-Henken / Biomed Pharmacother 56 (2002) 471–482
This is another ‘condition’ where arginine may become
virtually all tissues including the kidney, brain, liver, skel-
etal muscle and lungs However, the purpose of this
One of the major functions of arginine within the body is
as an intermediate in the urea cycle. The free amino acid
Another function of arginine is protein synthesis. Argin-
arginine contains a guanidino group, which is essential for
ine can be converted into proline, glutamate and glutamine
the synthesis of urea in most mammals. Urea is primarily
(see all of which are common amino acids found
synthesized in the liver and excreted by the kidneys. In the
within most proteins. Synthesis of these three amino acids
cytosol of hepatocytes, arginase-I removes the guanidino
begins with arginine being converted into ornithine. Orni-
group from arginine to produce urea and ornithine (see
thine is then converted into pyrroline-5-carboxylate. The
Urea is then transported from the hepatocyte into the
enzyme for this reaction is ornithine aminotransferase.